

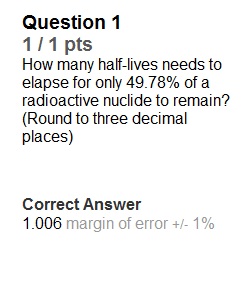
Q Question 1 1 / 1 pts How many half-lives needs to elapse for only 49.78% of a radioactive nuclide to remain? (Round to three decimal places) Question 2 1 / 1 pts In the lab, you have 518 g of a radioactive nuclide with half-life of 12.5 hrs. After some time, only 88.1 g of the nuclide remain. How many hours was the nuclide decaying? Question 3 1 / 1 pts You find a container in your lab with 348.7 grams of a radioactive nuclide with half-life of 5.8 minutes. How many grams of the nuclide will remain after 3.2 hr? Question 4 1 / 1 pts You find 862.5 g of a radioactive nuclide in the lab. 2.76 days later, only 283.4 g remains. What is the half-life (in hours) of the nuclide? Question 5 1 / 1 pts A certain radioactive isotope was allowed to decay for 7 half-lives. What percent of the original nuclide will remain? (round your percentage to 4 decimal places)
View Related Questions